
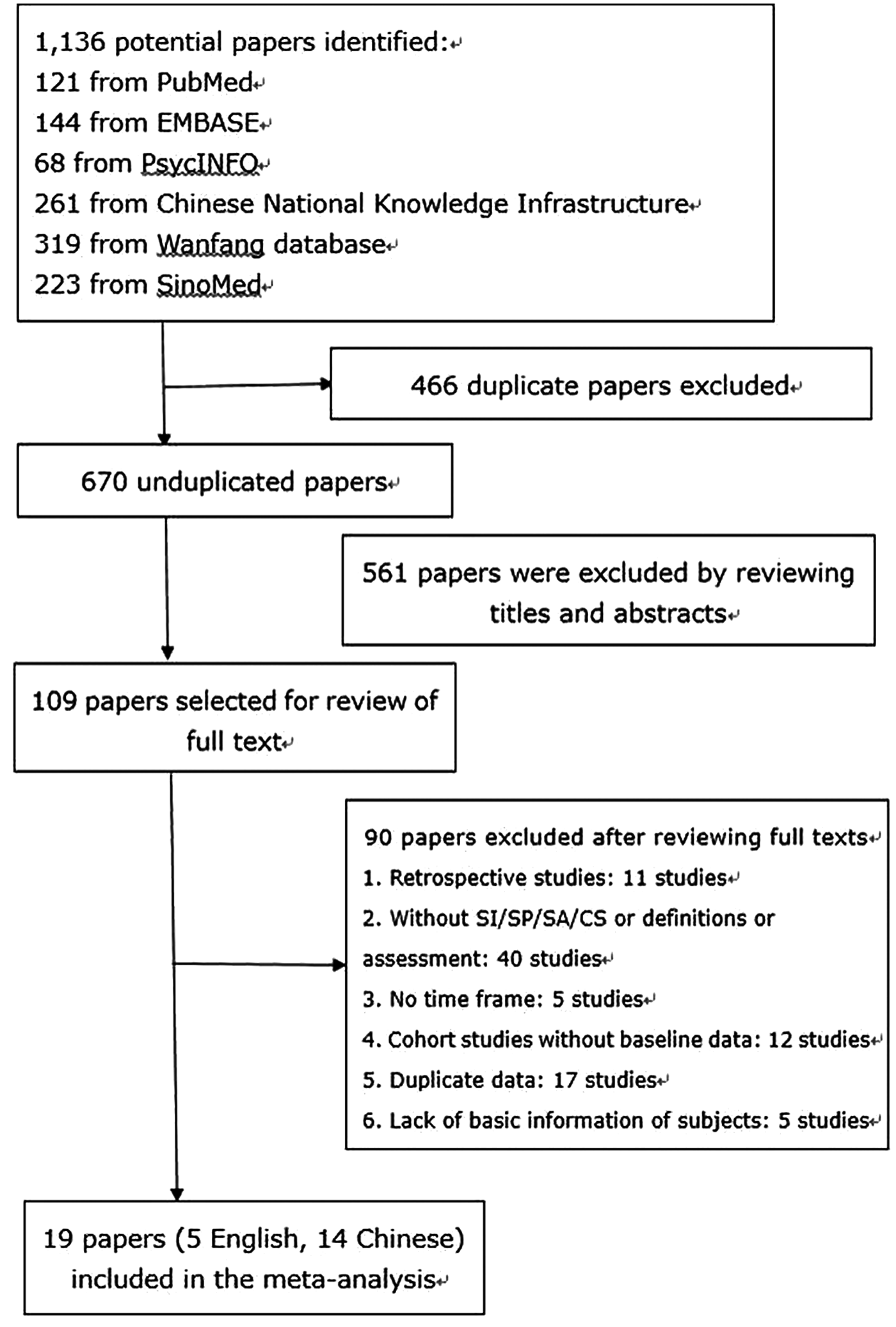
- How many studies for a comprehensive meta analysis review how to#
- How many studies for a comprehensive meta analysis review manual#
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D.

Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. The documentation provides a clear overview of scoping reviews. Use PRISMA-ScR, a 20-item checklist, for reporting scoping reviews. The documentation contains an excellent rationale for completing a protocol, too. PRISMA-P is a 17-item checklist for elements considered essential in protocol for a systematic review or meta-analysis. PRISMA may also be useful for critical appraisal of published systematic reviews, although it is not a quality assessment instrument to gauge the quality of a systematic review.Ĭonsider using PRISMA-P when completing your protocol. A 27-item checklist, PRISMA focuses on randomized trials but can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. Using the approriate standard can reassure editors and reviewers that you have conscienciously carried out your review.

You will improve the quality of your review by adhering to the standards below. Check here for templates, reporting standards, screening tools, risk of bias assessment, etc. Not a guide or standard but a clearinghouse for all things systematic review. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. Systematic reviews to answer health care questions

How many studies for a comprehensive meta analysis review how to#
Systematic reviews to support evidence-based medicine how to review and apply findings of healthcare research Provides a succinct outline for carrying out systematic reviews and well as details about constructing a protocol, testing for bias, and other aspects of the review process. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care They address the entire systematic review process, from locating, screening, and selecting studies for the review, to synthesizing the findings (including meta-analysis) and assessing the overall quality of the body of evidence, to producing the final review report. The IOM standards promote objective, transparent, and scientifically valid systematic reviews. Institutes of Medicine Standards for Systematic Reviews Very good chapters on conducting a review, most of which were published as articles in the Journal of Clincal Epidemiology. Methods Guide for Effectiveness and Comparative Effectiveness Reviews JBI maintains a page with other materials for scoping reviewers. Their chapter on scoping reviews provides a succinct overview of the scoping review process. The Joanna Briggs Institute provides extensive guidance for their authors in producing both systematic and scoping reviews.
How many studies for a comprehensive meta analysis review manual#
Scoping Reviews, JBI Manual for Evidence Synthesis Chapter 6, Searching for Studies, is most helpful in planning your review. The Cochrane Handbook isn't set down to be a standard, but it has become the de facto standard for planning and carrying out a systematic review.


 0 kommentar(er)
0 kommentar(er)
